Understanding the COVID-19 vaccine distribution
The current COVID-19 Vaccine US Supply Chain can be separated into two segments, each within the control of distinct decision makers. A schematic representation of the supply chain controlled by the Federal government is:
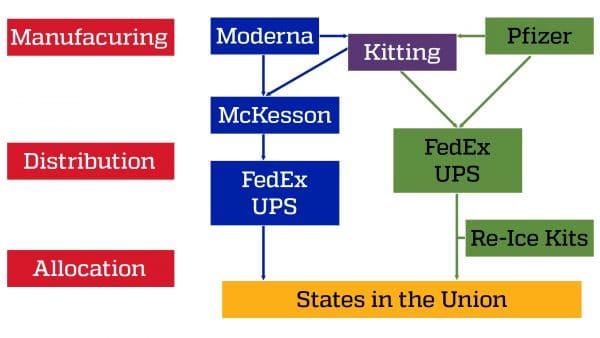
The U.S. Department of Health & Human Services also has this helpful chart to visualize the process.
In order to allocate vaccines to states, the process is as follows. Weekly allocations are provided to states on Tuesdays; after doses are ordered by states, shipments begin the following Monday. The entire order may not arrive in one shipment or on one day, but over the course of the week, delivery sites are notified by the private shipping partners. The Department of Health & Human Services also has more information.
For the first 4 weeks (December 14, 2020-January 10, 2021), the outcomes of this allocation process followed by the Federal government is summarized in the following table.
| State | US Pop (2019) | % | Pfizer First Dose | % | Moderna First Dose | % | Total First Doses | % |
| California | 38,642,700 | 12.10% | 1,323,075 | 11.11% | 1,368,600 | 11.23% | 2,691,675 | 11.17% |
| Florida | 20,992,000 | 6.58% | 724,425 | 6.08% | 748,700 | 6.14% | 1,473,125 | 6.11% |
| New York | 18,908,300 | 5.92% | 683,475 | 5.74% | 704,500 | 5.78% | 1,387,975 | 5.76% |
| Texas | 28,286,200 | 8.86% | 906,750 | 7.61% | 937,200 | 7.69% | 1,843,950 | 7.65% |
| Others | 212,420,100 | 66.54% | 8,273,850 | 69.46% | 8,429,900 | 69.16% | 16,703,750 | 69.31% |
| Total | 319,249,300 | 100.00% | 11,911,575 | 100.00% | 12,188,900 | 100.00% | 24,100,475 | 100.00% |
You also can learn more about population data or vaccine distribution data.
Examining the data in this table, some summary observations:
- It is fairly obvious that states are receiving first doses of each vaccine type in proportion to the population density within a state.
- In making these first dose allocations to each state, the Federal government is holding the second dose of each vaccine in reserve to be supplied at a later date. For example, the federal government is holding 1,323,075 and 1,368,600 doses of the Pfizer and Moderna vaccines, respectively, in stock for future shipment to California as a second dose.
- The total vaccine doses allocated to states during these first four weeks are approximately 24 million.
The second aspect of the vaccine distribution supply chain is completely under control of individual states. Vaccine doses are delivered by FedEx and UPS to locations specified by state regulators. The federal government announced January 12 that it recommends states work with pharmacies and community health centers to distribute the vaccine.
At this point, however, there is a remarkable inconsistency in how individual states split their allocations between different locations, as well as the mechanisms employed at each location to vaccinate individuals. For example:
- In California, initial vaccines were allocated to hospitals and long-term care facilities. There are plans to open large vaccination sites (e.g., the Disneyland Resort in Anaheim and Dodger Stadium in Los Angeles). There are also plans to utilize mobile clinics to distribute vaccines, and possible partnerships with colleges, universities and correctional facilities to ensure younger and incarcerated Californians have an opportunity to get a vaccine.
- In Florida, vaccines are available only at certain hospitals and long-term care facilities and through county health departments. Florida plans to set up mass vaccination clinics and may partner with pharmacies, community health centers and correctional facilities. Some counties have already set up drive-through vaccine sites similar to those currently used for COVID-19 testing. More recently, the state has made available vaccinations through partnerships with commercial retail chains (e.g., Publix).
- In New York, vaccines are available in hospitals and long-term care facilities. The state offers an online tool to help determine eligibility and vaccination sites across the state.
- The week of January 11, Texas will direct most COVID-19 vaccines received to large sites or hubs around the state to vaccinate more than 100,000 people. The state directs residents to consult the COVID‑19 Vaccination Hub Providers page and the Texas COVID‑19 Vaccine Availability map.
Summary Observations (December 14, 2020 – January 10, 2021)
Since the vaccine supply chain is controlled by two separate (non-coordinated) entities – the federal government and the state governments, the process and data summarized above indicates the following.
First, assuming the current allocations accurately reflects the production rate of vaccines by Pfizer and Moderna, each manufacturer’s capacity is approximately 24 million total doses (first and second) per month. Hence, if both manufacturers can maintain this output rate, then by the end of February 2022, the total number of doses allocated to states would be approximately 624 million. With each person requiring two doses of the vaccine, and assuming the Federal government maintains allocations based on state populations, there will be an adequate supply at the state level for inoculating the entire population in each state. If this is a reasonable time frame, then the two manufacturers should be monitored and incentivized to maintain their current production levels.
Second, there are aspects to concern as to how states are able to reach their populations to provide the vaccine as reflected in the following anecdotal evidence: (a) Of about 2.3 million coronavirus vaccine doses available in California, only 584,000 have been administered; (b) Of the more than 1.6 million doses of COVID-19 vaccines given to the state of Florida, only about 633,000 have been administered; (c) New York State has administered nearly 450,000 of the 1.4 million COVID-19 vaccines received; and (d) As of January 8, Texas has administered 527,533 first doses of COVID–19 vaccines developed by Pfizer and Moderna, which the Food and Drug Administration (FDA) has approved.
This analysis leads to the following prescriptive guidelines for inoculating the entire US population as quickly as possible.
- Monitor the vaccine manufacturers (Pfizer and Moderna) to ensure that they continue to provide the vaccine doses at current rates. Since Pfizer operates its own manufacturing supply chain, they could be in a better position to achieve their targeted rate. Moderna, however, is a new entrant in the manufacturing supply chain and relies on subcontractors and thus, monitoring their output rate is relatively more critical.
- If the current deadline of February 2022 is not acceptable, the Federal government should devise an incentive structure to motivate the current two manufacturers to ramp-up their current capacity. Of course, there is also the possibility of new vaccines entering the market (e.g. J&J) which could alleviate this problem.
- There is the need for federal intervention (and investment) to ensure that U.S. residents are quickly inoculated. The current inoculation/allocation rates within state are averaging 50% and this is leading to unnecessary delays. All in all, a centralized process is the appropriate solution in this context since not only are we facing a pandemic which is life-threatening, but it is also straining our medical resources. In addition, there is also some evidence that political considerations are influencing the inoculation process. Thus, prescriptive suggestions as we look ahead are as follows:
- Standardize eligibility criteria for vaccinations across states. This will require revisiting the allocation process of doses to states which will now be based demographic characteristics of the target population eligible for vaccinations.
- Establish mass inoculation sites at all major population centers in the U.S. (regardless of state). At each site, organize a personnel and supply infrastructure (in collaboration with state regulators) so that vaccine allocations to these sites are delivered to residents quickly.
- Directly coordinate with major medical centers in each state to establish vaccination sites.
- Expand reach in smaller population centers through partnerships with commercial firms (pharmacies, major retailers, etc.).